Monday, September 8, 2008
Chapter 5 Review
2. a
3. c
4. b
5. b
6. d
7. a
8. I might consume a big bowl of pasta because I want to store energy for the race and not to be too nervous.
9. Glucose, sucrose, and starch are all saccharides, and they follow the same chemical structure.
10. Steroids are lipid molecule with four fused carbon rings. One type of steroid circulate in our body as chemical signals, and the other one are the steroids estrogen
11. Proteins and polypeptides are both fromed by amino acids linking together.
12.
14.a) H2O
b) It's a chemical reaction, becuase when two amino acids join together, they from a new substance
c) The front part and the back part
15.a) Enzyme A performs best at about 37 ℃. Enzyme B performs best about 77℃
b) Enzyme A is in humans, and Enzyme B is in thermophilic bacteria.
c) IF a huaman's body temperature is above 40℃, that person must have caught a fever, enzyme only works in certain temperature. 40℃ is too hot for enzyme.
Thursday, September 4, 2008
5.5 Enzymes are proteins that speed up specific reactions in cells

- molecules absorb energy
- the "start-up' energy is called activation energy(ex: burn candle with match)
- heat up the mixture of molecules can provide activation energy
- catalysts, which cellular reactions depend on, are compounds that speed up chemical reactions

- enzymes are specialized proteins which are the main catalysts of chemical reactions in organisms
- enzymes can provide reactions to occur at the cell's normal temperature
- enzyme lowers the energy requirement barrier
- every enzyme catalyzes a specific kind of chemical reation
- the shape of each enzyme fits the shape of only particular reactant molecules
- a specific reactant acted upon by an enzyme is called the enzyme's substrate
- the substrate fits into a particular region of the enzyme, called the active site
- the fit between substrated and enzyme isn't rigid
- an znzyme can lower activation energy by accepting two substrates into adjacent sites
- enzymes can catalyze larger molecules from smaller molecules
- an enzyme's structure and shape are essential to its function
- an enzyme's shape is able to change it's surrounding environment
Concept Check:
1.Explain the role of activation energy in a reaction. How does an enzyme affect activation energy?
Activation energy activates the reatants and triggers a chemical reaction. An enzyme can keep a cell at its normal temperature, but you need to heat up molecules to provide activation energy.
2.Describe how a substrate interacts with an enzyme.
A substrate is a specific reactant acted upon by an enzyme, and substrate can help enzyme to lower its activatoin energy.
Tuesday, September 2, 2008
5.4 Proteins perform most functions in cell
The Functions of Proteins
1.protein is a polymer constructed from a set of just 20 kinds of monomers called amino acids
2.proteins are responsible for almost all of the day to day functioning of organisms
3.less-visible functional proteins circulate in the blood and defend body from harmful organisms
Amino Acids 
1.amino acid monomer consists of a central carbon atom bonded to four partners
2.three of the central carbon's partners are the same in all amino acids
3.four partners: one hydrogen atom, two carboxyl group and one amino group
4.the side group of the amino acids attracts water
Building a Protein
1.cells create proteins by linking amino acids together into a chain called a polypeptide 
2.each in link is created by a dehydration reaction between the amino group of one amino acid and the carboxyl group of the next amino acid in the chain
3.most polypeptide chains are at least 100 amino acids in length
Protein Shape
1.a protein in the simple form of amino acids linked together cannot function properly
2.a protein's shape is also influenced by the surrounding environment
3.an unfavorable change in temperature, pH, or some other quality of the environment can cause a protein to unravel and lose its normal shape
Concept Check:
1. Give at least two examples of proteins you can "see" in the world around you. What are their functions?
Human's hair and muscles. Hair and muscles of humans provide long-term nutrient storage.
2. Relate amino acids, polypeptides, and proteins.
Polypeptides are chains linked by amino acids, and proteins are polypeptide molecules.
3. Explain how heat can destroy a protein.
Heat can make an unfavorable change in temperature, so the change can cause a protein to unravel and lose its normal shape.
4. Which parts of an amino acid's structure are the same in all amino acids? Which part is unique?
All amino acid consist of a central carbon bonded to an amino group, a carboxyl group, and a hydrogen atom. The side groups.
Monday, September 1, 2008
5.3 Lipids incluede fats and steroids
2.hydrophobic is molecules that are water-avoiding

The fats in fruits, vegetables,fish are unsaturated fat. Unsaturated fat is more healthier to our body.
5.1 Carbon is the main ingredient of organic molecules


5.2 Carbohydrates provide fuel and building material Summary
1.sugar molecules made up an organic compound called carbohydrate
2.sugars contain 1 carbon: 2 hydrogen: 1 oxygen.
3.any carbohydrate's molecular formula is : CH2O
4.most sugar moleculs in nature are ring shape carbon skeletons
5.monosaccharides are simple sugars that contain just one sugar unit
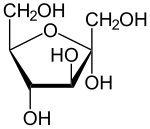
6.sugar molecules, particularly glucose, are main fuel supply for cellular work
7.carbon skeletons of monosaccharids are used as raw material for manufacturing
other kinds of orgainc molecules by cells
8.disaccharide = ' double sugar'
 ( ex: sucrose)
( ex: sucrose)9.sucrose consists a glucose moleculed linked to a fructose molecule
10.sucrose is a major carbohydrate in plant sap
11.body store glucose in larger molecules for later use
Polysaccharides:
1.long polymer chains made up of simple sugar monomers are called polysaccharides
2.starch is a polysaccharides being found in plant cells that consists only glucose monomers
3.the stored glucose becomes available when plants break down starch molecules
 ( ex: foods rich in starch)
( ex: foods rich in starch)4.glycogen is a form of polysaccharides which is the way animals such as turkeys and humans
store excess sugar
5.glycogen is highly branched than a starch polymer
6.cellulose is a kind of polysaccharides that's in plants which serve as building materials
7.cellulose protect cells, stiffen the plant, and prevent it from flopping over
8.most animals, including people, cannot digest cellulose
9.almost all carbohydrates are hhydrophilic
10.monosaccharides and disaccharides dissolve in water and form suagary solutions

Concept Check
1. Explain the difference between a monosaccharide and a disaccharide. Give an example of each.
A monosaccharide is sugar that contain one sugar unit, a disaccharide has double sugar which formed by two monosaccharide
2.Compare and contrast starch, glycogen, and cellulose.
Starch, glycogen, and cellulose are all polysaccharide. Starch is in plant cells, glycogen is in animal cells, cellulose is in plants and are served as building materials.
3.How do animals store excess glucose molecules?
By incorporate excess glucose molecules into larger carbohydrates, or the excess glucose molecules may be used to make fat molecules




