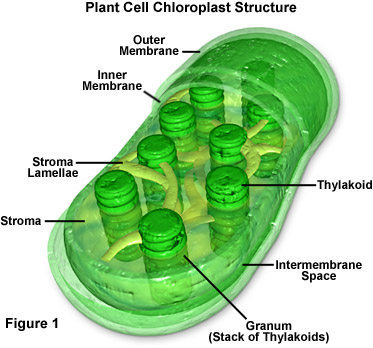
- carbon cycle that occur on a global scale on Earth depend on the metabolism of tiny chloroplasts and mitochondria
- both producers and consumers' cellular respiration returns carbon dioxide to the atmosphere
- other chemical process on the planet doesn't match the output of photosynthesis
- carbon dioxide is the main element of the carbon cycle
- during cellular respiration, most organisms give off carbon dioxide as wastes
- all the organisms on Earth has a very large effect on the amount of carbon dioxide in the
 atmosphere
atmosphere - greenhouse effect, carbon dioxide in the atmosphere traps heat from the sun that would otherwise escape from Earth back into space, which keeps the world climate warm enough for living things
Voabulary:
- carbon cycle - process by which carbon moves from inorganic to organic compounds and back
- greenhouse effect - process by which atmospheric gases trap heat close to Earth's surface and prevent it from escaping into space
Concept Check:
- It helps to keep the temperature on earth warm enough for organisms.